Abstract
Background and aim: Most frequent and generally unpredictable coronary plaque rupture impacts the burden of coronary artery disease but features or signs related to plaque remodeling into the high risk structure are not clearly detectable by using ordinary visualization methods. Till yet there are no evident criteria for additional using IVUS. The aim of the study was to determinate intravascular ultrasound virtual histology (IVUS-VH) importance in identifying high risk plaques, which can contribute to increased rupture hazard. Methods: We selected 30 patients with stabile angina pectoris. 50 plaques were analyzed with coronary angiography digital assessment tool and IVUS similarly. Differences of stenoses measured by both methods, then were calculated and compared to composition of plaques evaluated by IVUS-VH. Results: Plaques were mostly formed of fibrous tissue (FI) (2.6 mm2; 57.89 %). Necrosis was found to make in average one-fifth of analyzed plaques (0.75 mm2, 19.60 %). Calcification made up the smallest part of plaques (0.3 mm2, 8.58 %). Plaques with higher necrosis component appeared to be significantly greater in IVUS compared to coronary angiography. In group A necrosis made up 1.40±1.05 mm2; group B – 0.87±0.52 mm2, and group C – 0.62 ±0.45 mm2 (0.020). The same tendency was observed with FI: group A – 3.38±3.20 mm2; group B – 2.90±2.6 mm2 and group C – 2.04±165 mm2 (0.082). Correlation analysis revealed negative moderate relationship between groups and necrosis percentage (–0.40, 0.004), and FI ( –0.29, 0.039) components of the plaques. Conclusion: IVUS-VH provides new insight into the evaluation of different composition of plaques. However, despite the advantages, IVUS-VH remains costly and not always technically adaptive procedure, so it is necessary to pursue for new methods or technologies to identify atherosclerotic plaques at risk.
1. Introduction
Atherosclerosis is the main cause of coronary heart disease (CAD), which is currently leading cause of death worldwide and claim to be the first in 2030 [1-3]. Most frequent and generally unpredictable coronary plaque rupture impacts the burden of CAD [4]. However, despite of importance features or signs related to plaques remodeling into the high risk structure prone to rupture are not clearly detectable with ordinary visualization methods. Commonly in clinical practice used coronary angiography represents coronary arteries as a planar picture of lumen (Fig. 1(a)). Processed visual screening allow to show artery diameter reduction (stenosis) in comparison with normal arterial segment, approximately calculate stenosis size, in accordance to ordinarily described rate: normal (< 25 %), low grade (25-49 %), intermediate grade (50-74 %), high grade (75-90 %), subtotal (91-99 %) and total occlusion (100 %). However, this does not take possibility accurately and completely to visualize structure of coronary artery wall, obtain clear orthogonal segmental views and herewith avoid hiper/hipo misclassification or other diagnostic mistakes. Errors can also arise from misclassification due to coronary spasm, coronary arteries anomalies, and deep intubation of ostial stenoses, poststenotic dilatation, or (every so often) inadequate judgment by the operator [5]. Therefore, coronary angiography can’t be suitable for comprehensive clinical assessment of sclerotic plaque structure (Fig. 1(a)).
Fig. 1a) Typical view of CAA (Place of stenosis marked with black arrow); b) quantitative coronary angiography view; c) evaluation of the plaque using IVUS-VH; d) evaluation of the plaque using IVUS
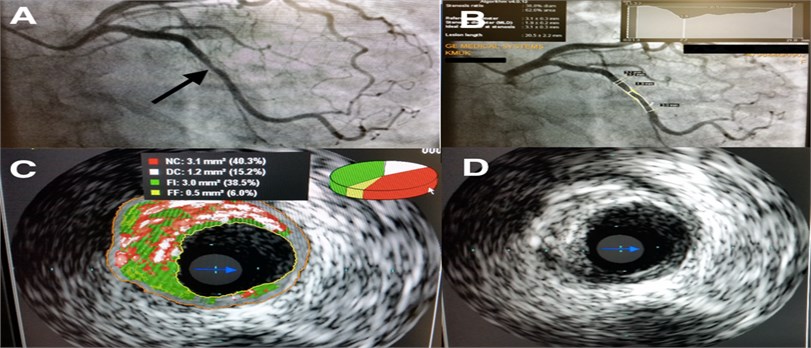
Quantitative coronary angiography is more recent, an automated, computer based alternative method outperforming analysis of two dimensional coronary angiographic images with automated contour detection (Fig. 1(b)). It is available for immediate online or post hoc offline analysis and allows partially avoid operator-related errors but thereby requires precise standardization of image acquisition to be reliable [5].
The ultimate in technology intravascular ultrasound (IVUS) similarly are intracoronary imaging method which enable allow true three-dimensional structural quantification of coronary arteries and sclerotic plaques, what is necessary and important in everyday clinical practice. IVUS provides to determine coronary vessel size, extent, location of plaque, what significantly facilitate therapeutic decision-making during the catheterization procedure. Though, IVUS not enable fully facilitate one of the essential sclerotic plaque structural component – presence of necrosis and its mass, which is known highly trombogenic, inflammable and considered important as plaque rupture prognostic factor. As alternative IVUS-VH (IVUS – Virtual Histology) is much more effective tool for plaque morphology and composition assessment and can be potentially important clinical implication for patients with acute coronary syndromes, unstable angina and high risk of sudden coronary death [6, 7]. Another reporting shows IVUS-VH method not so promising and has high accuracy only in detecting dense of calcium, what conversely represent stability of the plaque [8]. Problem is in part due to the fact that wide clinical studies using IVUS had serious limitations to include to the study a large population.
Aim of our study was to determinate specific criteria for IVUS-VH using and importance improving of this diagnostic method to identify vulnerable patients, and the plaques, which can contribute to increased rupture risk.
2. Methods
Study was performed in period of May 2013 and March 2015. We included patients (-30) admitted to the Clinic of Cardiology, Lithuanian University of Health Sciences, Clinical Hospital. All patients had diagnosis of stabile angina pectoris (SAP) with indications for coronary angiography. Initially, according to common protocol, intracoronary plaques were evaluated by quantitative method, described above. Then, in purpose to qualify plaque projection in all main coronary arteries was performed IVUS-VH test, simultaneously using the administration of glyceril trinitrate, to minimize possible vasospasm. IVUS procedure was acquired with Volcano s5 Intravascular Ultrasound Imaging System. Also we apply Eagle-Eye Platinum catheters (Volcano) using motorized pull- back at 0.5 mm/s from the most distal part of vessel. Data were captured on Volcano s5 imaging system and analyzed using software version 3.2.X (Volcano). Compositional data was obtained for every slice and expressed as mean percent for each component. Mainly we evaluated projection, grade of stenosis and structural composition of plaque. In case of established stenosis more than 75 % commonly we chose stent implantation, checking by IVUS optimal stent position or possible stenting complications (dissection and etc.). In cases of established stenosis less than 75 % we preferred adequate conservative treatment.
Statistical analysis was performed with IBM SPSS 17.0 software package. Data set were tested for normality distribution with Kolmogorov-Smirnov test (small samples were tested with Shapiro-Wilk test). For comparison of parametric quantitative variables non-paired Student’s -test and for non-parametric Mann-Whitney (U) test were used. More than two groups with normal distribution were compared with ANOVA (F) (LSD criterion), and in case without normal distribution we used Kruskal-Wallis test. Difference in frequency of qualitative variables was evaluated by chi-square () test. Correlation between data was assessed using Spearman correlation coefficient. The p value less than 0.05 was considered as statistically significant.
3. Results
Studied population consisted of 30 patients (20 (66.7 %) men) and mean age was 56.6±8.0 years. The main clinical characteristics of study population are presented in Table 1.
In total 50 detected atherosclerotic plaques were analyzed. Stenoses were determined and calculated by using coronary angiography digital assessment tool and ranged from 18.9 % to 88.4 % (mean 49.1 %±18.3 %), while assessed with IVUS-VH tool were 33.6 % to 73.5 % (mean 49.5 %±8.6 %). The differences between stenoses detected on coronary angiography and IVUS respectively were calculated and results were grouped according to percentile they were in: group A – data under 25 percentile – < –18.62 % (stenoses calculated with IVUS-VH tool were significantly higher compared to coronary angiography – 15 plaques, 30 %); group B – data in 25-75 percentile (difference of stenoses interval –18.62 % to 12.64 % – 23 plaques, 46 %); group C – data over 75 percentile > 12.64 % (stenoses calculated with IVUS-VH tool are significantly lower compared to coronary angiography – 12 plaques, 24 %).
Table 1Clinical characteristic of studied population
Characteristic index | |
Sex (men), (%) | 20 (66.7 %) |
Mean age (years) | 56.6±8.0 |
Arterial hypertension, (%) | 30 (100 %) |
Diabetes mellitus, (%) | 3 (10.0 %) |
SAP class (%) | |
II | 22 (73.3 %) |
III | 8 (26.7 %) |
Smoking, (%) | |
Non-smoker | 14 (46.7 %) |
Currently | 6 (20.0 %) |
Former smoker | 10 (33.3 %) |
Dyslipidemia, (%) | 27 (90 %) |
Obesity, (%) | 15 (50.0 %) |
Body mass index, kg/m2 | 30.9±5.9 |
The composition of plaques was evaluated by presence of standard dimensions: necrosis, calcification, fibrous (FI), and fibrous fatty (FF) tissue. Components were analyzed by their absolute area in plaque and their percentage distribution (Table 2). We determined that plaques were mostly formed of fibrous tissue (2.6 mm2; 57.89 %). Necrosis was found to make in average one-fifth of analyzed plaques (0.75 mm2, 19.60 %). Calcification made up the smallest part of plaques (0.3 mm2, 8.58 %).
Table 2Composition of atherosclerotic plaques
Characteristics | |
Necrosis, mm2 | 0.75 (0.48-1.23) |
Calcification, mm2 | 0.30 (0.10-0.51) |
Fibrous tissue, mm2 | 2.60 (1.48-3.60) |
Fibrous fatty tissue, mm2 | 0.57 (0.31-1.03) |
Necrosis, % | 19.60±7.77 |
Calcification, % | 8.58±6.9 |
Fibrous tissue, % | 57.89±9.55 |
Fibrous fatty tissue, % | 13.92±6.72 |
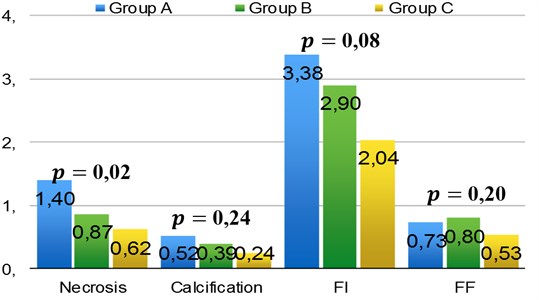
The comparison of plaque components absolute area values in three groups showed significant difference in necrosis and FI (Fig. 2). Plaques with higher necrosis component appeared to be significantly greater in IVUS-VH comparing to coronary angiography. In group A it consist 1.40±1.05 mm2; group B – 0.87±0.52 mm2 and group C – 0.62±0.45 mm2 (0.020). The same tendency was observed with FI: group A consist 3.38±3.20 mm2; group B – 2.90±2.6 mm2 and group C – 2.04±1.65 mm2 (0.082). No significant differences were observed when percentage distribution of components in plaques were compared (0.05).
Correlation analysis revealed negative moderate relationship between groups and necrosis percentage ( –0.40; 0.004), and FI ( –0.29; 0.039) components of the plaques. The stenoses calculated with coronary angiography appeared lower compared to IVUS-VH when necrosis or FI parts in plaque were significantly greater.
Fig. 2Distribution of necrosis, calcification, FI and FF in atherosclerotic plaques, FI – fibrous tissue; FF – fibrous fatty tissue
4. Discussion
Trying to completely understand the atherosclerosis process with respect to the pathophysiology of acute coronary syndromes (ACS) has continued till now. ACS is often the first manifestation of coronary artery atherosclerosis, making accurate and timely identification of potentially vulnerable plaques important at high risk patients. Today common cardiac intervention practice essentially focuses to coronary angiography as “gold standard” on regardless to identify presence of atherosclerotic plaques. Another frequently used invasive methods for more correct coronary artery stenosis assessment are intravascular ultrasound (IVUS) and fractional flow reserve (FFR). There IVUS can provide accurate anatomical information, whereas FFR more assesses the functional or physiological significance of a lesion and is more a physiological parameter that can discriminate the presence of myocardial ischemia. Some studies have shown a good correlation between FFR and minimum lumen area by IVUS. But in proximal lesions, IVUS criteria such as lumen area of 3 to 4 mm2 and/or percent area stenosis of 70 % to 80 % are reported to accurately predict the functional significance of intermediate coronary stenoses [14, 15]. Our study also demonstrates no significant statistical differences between IVUS and standard coronary angiography in detecting average range of the stenosis: mean 49.5 % and 49.1 % respectively, although in absolute rating it differs markedly. However, IVUS is commonly done to make sure a stent is correctly and optimally placed especially during complicate angioplasty. IVUS also can be important observing internal changes of the coronary arteries – such as evaluating the role of vulnerable plaque and the impact of cholesterol lowering medications on regression. Undoubtedly, IVUS-VH provide markedly more information in the field of coronary angiography seriously extending diagnostic and prognostic possibilities evaluating high risk plaques and determine the need for aggressive management of risk factors prior to onset of symptoms and advanced disease [11].
We have a notion that particular plaque instability characteristics, such as large necrotic core, thin fibrous cap, varied mechanical properties of the plague can have significant clinical importance, what is often ignored. Some studies have indicated the correlation between plaque characteristics and plaque vulnerability [13]. In a post-mortem validation study, IVUS-VH analysis demonstrated high sensitivity and specificity for detection of necrotic core of 92 % and 97 % respectively [3]. IVUS-VH has also been developed to detect “necrotic core,” a term considered to be synonymous with “lipid core” [12]. We also agree with point that plaque burden > 40 %; confluent necrotic core > 10 % in direct contact with the lumen in the investigated cases are appreciate as a having high-risk to rupture [10] and need IVUS (VH) testing. The findings in our study demonstrate presence of plaques with high necrosis component in one fifth of analysed plaques concerning about 19.6 % what means presence of high risk plaque. We also agree with point of PROSPECT study demonstrating that IVUS and IVUS-VH technologies can assess risk of a clinical event better than using coronary angiography alone.
However, we must talk about some limitations of this method. At first, detection of thin fibrous caps < 65 μm in thickness is below the resolution of the technology. As an IVUS-derived technique, its axial resolution also is limited [5]. Diagnostic accuracy is higher in lesions at the proximal LAD, which usually has fewer anatomic variations and significantly lowers in segments with relatively higher anatomic variations. Second, IVUS catheters is enough large, approximately 1.5 mm in diameter, consequently is possible examine only wide segments of the main coronary arteries, but still not all especially distal segments of interest. Third, IVUS-VH does not provide information on thrombus and inflammation. And finally, using of this system is enough highly priced at yet, therefore its not be available to perform all necessary coronary arteries examinations. We agree with opinion what reduced cost has led to more personalized medicine.
5. Conclusions
Coronary angiography remaining as the “gold standard” is not a good predictor of future events. Using of IVUS technologies is necessary because of assessment risk of clinical outcomes better than using coronary angiography alone. IVUS diagnostic accuracy is higher in lesions at the proximal LAD and significantly lowers in segments with relatively higher anatomic variations. Despite advantages IVUS at yet remains costly and not always technically adaptive procedure, so it enforce pursue the new methods or technologies identify atherosclerotic plaques at risk.
References
-
http://www.who.int/whosis/whostat/EN_WHS08_Full.pdf
-
Detailed work for everyone, especially the section covering the anatomy of normal coronary arteries as well as the anatomy of coronary arteries in disease is highly recommended. This monograph may no longer be available but is integrated in Braunwald’s Heart Disease. A Textbook of Cardiovascular Medicine, 1980.
-
Nair A., Margolis M. P., Kuban B. D., Vince D. G. Automated coronary plaque characterisation with intravascular ultrasound backscatter: ex vivo validation. EuroIntervention, Vol. 3, 2007, p. 113-20.
-
Burke A. P., Kolodgie F. D., Farb A., Weber D. K., Malcom G. T., Smialek J., Virmani R. Healed plaque ruptures and sudden coronary death: evidence that subclinical rupture has a role in plaque progression. Circulation, Vol. 103, 2001, p. 934-940.
-
http://www.pcronline.com/eurointervention/textbook/pcr
-
Nasu K., Tsuchikane E., Katoh O., Vince D. G., Virmani R., Surmely J. F., Murata A., Takeda Y., Ito T., Ehara M., Matsubara T., Terashima M., Suzuki T. Accuracy of in vivo coronary plaque morphology assessment: a validation study of in vivo virtual histology compared with in vitro histopathology. Journal of the American College of Cardiology, Vol. 47, 2006, p. 2405-2412.
-
Calvert P. A., et al. VH-IVUS and MACE in coronary artery disease. JACC: Cardiovascular imaging. Vol. 4, Issue 8, 2011, p. 894-901.
-
Nair M. P. A., Kuban B. D., Vince D. G. Automated coronary plaque characterization with intravascular ultrasound backscatter: ex vivo validation. Eurointervention, Vol. 3, 2007, p. 113-121.
-
Jang I. K., Tearney G. J., Macneill B., Takano M., Moselewski F., Iftima N., Shishkov M., Houser S., Aretz H. T., Halpern E. F., Bouma B. E. In vivo characterization of coronary atherosclerotic plaque by use of optical coherence tomography. Circulation, Vol. 111, 2005, p. 1551-1555.
-
García-García H. M., et al. Virtual histology and remodelling index allow in vivo identification of allegedly high-risk coronary plaques in patients with acute coronary syndromes: a three vessel intravascular ultrasound radiofrequency data analysis. EuroIntervention, Vol. 2, 2006, p. 338-344.
-
Nair A., Kuban B. D., Tuzcu E. M., Schoenhagen P., Nissen S. E., Vince D. G. Coronary plaque classification with intravascular ultrasound radiofrequency data analysis. Circulation, Vol. 106, 2002, p. 2200-2206.
-
Brugaletta S., et al. NIRS and IVUS for characterization of atherosclerosis in patients undergoing coronary angiography. Journal of the American College of Cardiology: Cardiovascular Imaging, Vol. 4, Issue 6, 2011, p. 647-655.
-
Motoyama S., Sarai M., Harigaya H., Anno H., Inoue K., Hara T., Naruse H., Ishii J., Hishida H., Wong N. D., Virmani R., Kondo T., Ozaki Y., Narula J. Computed tomographic angiography characteristics of atherosclerotic plaques subsequently resulting in acute coronary syndrome. Journal of the American College of Cardiology, Vol. 54, Issue 1, 2009, p. 49-57.
-
Takagi A., Tsurumi Y., Ishii Y. Clinical potential of intravascular ultrasound for physiological assessment of coronary stenosis: relationship between quantitative ultrasound tomography and pressure-derived fractional flow reserve. Circulation, Vol. 100, 1999, p. 250-255.
-
Briguori C., Anzuini A., Airoldi F. Intravascular ultrasound criteria for the assessment of the functional significance of intermediate coronary artery stenoses and comparison with fractional flow reserve. American Journal of Cardiology. Vol. 87, 2001, p. 136-141.
