Abstract
Bruxism as a pathophysiological entity, both day and night bruxism, has been the subject of innumerable investigations. Among the probable causes that have been raised are biochemical alterations in the central and/or peripheral nervous system, which can be seen reflected in an alteration of the rhythmic movements of the masticatory musculature of the stomatognathic system. On the other hand, chewing is recognized as one of the main functions of the stomatognathic system, responsible for maxillofacial growth and determining a rhythm in the movements of the masticatory musculature, depending in turn on a functional demand of the individual, in other words, the type of food consumed and the way to process it in the mouth. Objective: To determine the existence of a relationship between bruxism and the type of masticatory pattern installed in the patient. Methodology: Functional examinations and application of Fonseca questionnaires and bruxism self-report were performed in 27 adult patients, without distinction of sex, who have been diagnosed with possible and probable bruxism. Results: 100 % of the sample of subjects diagnosed with bruxism presented an altered masticatory pattern. Conclusions: The high correlation found in the sample between bruxism and masticatory pattern suggests that it is vital for the success of a bruxism treatment or a DTM to consider the way of processing food, by educating the patient with Masticatory Orientation. Based on the above, it proposes to conduct a study to evaluate the efficiency of a functional treatment for future Bruxism therapies.
1. Introduction
The human body and its functioning are the result of the harmonious interaction of its different systems, therefore, if any of them present an alteration, this harmony and its functioning will be affected. The stomatognathic system (S.S), as one of the systems of the human being, is defined as the morphofunctional unit or biological system which is anatomically located in the cranio-cervical-facial territory, basically, involving the combined structures of the mouth, and the jaws, it develops multiple functions which affect its environment (musculature, teeth, bone tissue, temporomandibular joint) [1, 2]. Among the most important functions are suction, swallowing, phonoarticulation, chewing and precise coordination with breathing through the installation of an oral valve system. These functions, which make up the S.S, disseminate a large amount of information and stimuli over the different tissue, which are reflected in the growth and development of the system itself. It includes the physiological wear of all its components, both hard and soft, teeth with physiological wear, the bone tissue in permanent remodeling, installation of patterns or neuromuscular engrams of masticatory and swallowing muscles, all of those as a response to a functional demand that, the more physiological demands it requests, the greater the development achieved by the S.S [2, 3]. When the functional demand is minimal, the harmony between function and development begins to decrease. Consequently, the usual parafunction may appear, but normally it does not harm the S.S in a first instance, as long as they do not exceed the capacity of adaptation and resistance of the tissues to overexertion or continuous functional overdemands. This condition is considered as a risk factor for disorders such as temporomandibular disorders (TMD), bruxistic activities and other pathologies.
1.1. Chewing
Chewing is one of the most principal functions for the stomatognathic system. This consists of the act of biting, crushing and chewing food as homogeneously as possible using the upper teeth against the lower ones, mobilizing the food from the right side to the left side and vice versa as many times as possible, this is considered a complex neurological process that also depends on the development of the craniofacial complex, of the central nervous system and dental occlusion [3-5]. The masticatory function is characterized by generating motor responses, which are rhythmic and learned, combining both reflex and voluntary activities. Once started, it continues almost automatically, being voluntary the start and end of the activity and being intentionally accelerated, slowed down or stopped.
The afferent sensory information of the S.E is conducted trigeminally to various structures of the central nervous system (CNS). This sensory information stimulates various areas within the brain stem and neocortex, both areas involved in important higher functions. The neuronal stimulus generated by chewing (considering all its stages) adding the times a day that it is triggered, takes approximately one hour. During this time, the different structures of the stomatognathic system are used thoroughly, and both the quality of this function and the excitation it produces, ultimately depend on the nature of the food used, so it must always be a food that stimulates a high functional demand [5-7].
Chewing is considered as a learned orofacial function and of relevance for the harmonious development of the stomatognathic and craniofacial system. As an alternating bilateral pattern, it promotes the symmetry of the neuromusculature, a harmonious growth of the maxillary bone bases, and which means a balanced occlusion with physiological wear. When the masticatory pattern is not physiological or ideal, an alteration of the temporomandibular joint may occur, with simultaneous contacts of work, rolling and sliding [8, 9]. In addition, chewing is an oral function that is closely related to the physical, mental and social health throughout the life of the individual.
Chewing has been tried to bring to an objective and quantifiable plane for study and analysis. One of the ways to objectively measure chewing is to consider masticatory efficiency, bite strength and ability to mix food, or it can be subjectively considering the perception of the patient’s own masticatory capacity. From the point of view of orofacial motor skills, the evaluation of chewing considers different aspects, such as: number of masticatory cycles, masticatory pattern, masticatory time, among others masticatory cycles correspond to the rhythmic movement that is generated as a result of the combination of movements when performing the masticatory drop, where the occlusal characteristics are important, in fact one of the conditions of occlusal stability is bilateral chewing with protrusion during incision [8-10].
1.2. Masticatory pattern (Pm)
Chewing is a motor and sensory function of the stomatognathic system, rhythmic, coordinated and learned, that is, it is stimulated and fed back by the peripheral perceptions of the stomatognathic system itself, its relationship with food and the entire environment of the masticatory process, involving the other organs of the senses such as smell, vision, touch and postural system. From all these areas they leave, by afferent routes, carrying the information to the Central Nervous System, from which efferent pathways emerge that go to different effector organs (muscles, tendons, glands) [11, 12] (Fig. 1).
Fig. 1Diagram of nerve transmission pathways
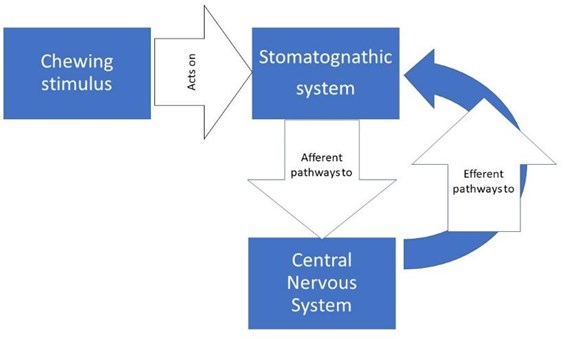
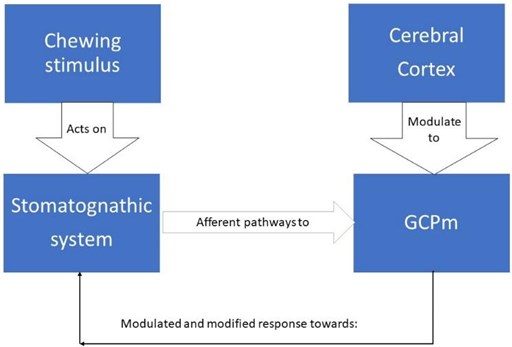
Neurologically this sensory stimulation and feedback activates a set of neurons responsible for the rhythm and coordination of chewing, located in the brainstem called the central generator of masticatory patterns (GCPm). The GCPm is found at the ponto-bulbar junction of the brainstem, specifically in the medial bulbar reticular formation between the motor root of the trigeminal nerve and the olive inferior. By definition, a pattern-generating center could produce a rhythmic activity in the absence of peripheral or upper descending stimulation, but this does not mean that peripheral stimulation does not play an important role in shaping the motor responses of that generating center , on the contrary, there is a great variability between each masticatory cycle and this is because the neurons of this generating center are constantly subjected to a feedback mechanism, which is born from the information emanated of the proprioception in the stomatognathic system and the higher centers such as the cerebral cortex, amygdala, periaqueductal gray matter, among others. This is important in activities of the human life such as how to manage emotions, instincts, discrimination between food, taste, hunger, salivary secretion and swallowing [13] (Fig. 2).
Fig. 2Scheme of variability in GCPm by feedback
The masticatory pattern, masticatory type or preferred side of chewing is defined as the location of the food bolus and the route of mandibular displacements during the masticatory act [13]. When establishing a measurement of the number of masticatory cycles in percentage to the right and left or both sides, the following relationship is had between 50 % and 65 % on each side is considered alternating bilateral chewing, more than 65 % on both sides is considered simultaneous bilateral chewing or hinge, between 66 % and 75 % on the same side is considered unilateral preferential and more than 75 % on the same side is considered chronic unilateral chewing. For efficient chewing it is considered that the alternated bilateral pattern turns out to be the ideal physiologically, which would imply that any other pattern could be considered as a chewing dysfunction with alteration of the efficiency, time and capacity of the masticatory process [14, 15].
Patients with temporomandibular disorder (TMD) usually present an altered masticatory function, it is thought due to direct factors including tooth pain or loss of teeth, and indirect factors such as joint damage or hypermobility that can alter masticatory function. From a more functional perspective, it is logical to think that, for some reason, the masticatory pattern was altered or was never learned correctly, what eventually would cause a functional alteration in the temporomandibular joint, and if it holds up over time, can become a structural injury [16].
1.3. Bruxism (Bx)
In 2017, the ninth edition of the glossary of clinical prosthodontic terms (GPT9) was published by the American Academy of Prosthodontics, where Bx is considered as parafunctional teeth grinding or clenching, a nonfunctional, rhythmic, spasmodic and involuntary oral habit [17, 18].
Bx is considered a parafunction of multifactorial origin, since neither its etiopathogenesis nor its pathophysiology is clear, being mainly regulated by the central nervous system (CNS) and influenced by peripheral factors. It is characterized by repetitive jaw movements with no apparent functional purpose, especially clenching and/or grinding of the teeth, severely damaging the S. It can cause extensive loss of dental tissue by attrition (bruxofacets) or abfraction, dental fractures, dental hypersensitivity, pain in the orofacial musculature, masticatory dysfunction and TMD, because the adaptive capacity of the E.S. is exceeded by the intensity of the forces exerted on its components [19-21].
The first cause of Bx remains unspecified, which has generated different theories regarding its origin and has been associated with multiple risk factors. The risk factors mentioned can be classified into 2 groups that can even be interacting: peripheral or morphological/anatomical factors, and central psychological/pathophysiological factors [22]. Dentoskeletal alterations correspond to peripheral, morphological or anatomical factors. Within this group we can mention the alterations in dental occlusion, in the presence of dentomaxillary anomalies and their relationship with the cranio-cervico-mandibular unit, a possibility based on the assumption that, in the absence of occlusal balance, the activation of periodontal receptors occurs, with a secondary response of reactive type. Peripheral alterations, more precisely morphological alterations, were considered for a long time as the main factor causing bruxism due to their prevalence, which was much higher in population groups with malocclusion (class II and III) than in comparable groups with normal occlusion, triggering an excessive activity of the mandibular muscles [23-26]. This led to the inference that the corrected or adjusted occlusal alteration was sufficient to cause the disappearance of teeth grinding. In this way the theory was established that through bruxism the organism tried to eliminate these occlusal interferences. Currently, clinicians have expanded the search for other causal factors, where dental occlusion yields its action to other areas of knowledge (psychological and pathophysiological factors). This demonstrates a tendency in bruxism research closer to a biomedical-biopsychosocial model/paradigm than only to occlusion, mainly because it has been observed that not everyone is involved bruxist presents occlusal interferences and not everyone with these interferences is a bruxist, but the same studies do not indicate anything about the masticatory pattern present in the patient with Bx [27-29] .The scientific literature has repeatedly shown that the theory of alterations in dental occlusion lacks scientific validity, possibly because of a problem of methodology rather than a problem of clinical outcomes [30-32].
In the various studies of patients with possible and probable bruxism or who present TMD, there is a total lack of analysis of the masticatory function and its neuromuscular pattern [33, 34], rather than the peripheral factors themselves, as a possible causal factor of Bx or how determinant a specific type of masticatory pattern may be on the type of occlusion, given that the neuromuscular activity demanded by the function is very complex and requires precise muscular coordination of agonists and antagonists, this has implications at the level of the CNS, neocortex and hypothalamus [35, 36], it should be remembered that thanks to neuromuscular activity the formation and remodeling of bone tissue began at the embryonic level, therefore a bone problem has its origin in a muscular problem and not the other way around.
Therefore, the present pilot study aims to determine the type of chewing pattern installed in bruxing patients and the possible existence of a relationship between the presence of bruxism, answering our research question: Is there a predominant chewing pattern in bruxing patients and, if so, what is it?
2. Materials and methods
A cross-sectional observational pilot study was designed with a purposive sample of 27 Chilean subjects diagnosed with possible or probable bruxism, with the presence of at least 24 teeth for the analysis, without distinction of sex, after signing an informed consent form. The study subjects were adults between 19 and 60 years of age, with an average age of 36.6 years old, with and without dentomaxillary anomaly. Subjects with previous phonoaudiological or orthodontic treatment, head and neck surgeries or the presence of craniofacial syndrome or malformation were excluded.
2.1. Masticatory evaluation protocol
The procedure for the clinical evaluation of the masticatory pattern was performed by a group of 5 calibrated evaluators under the guidance of an expert. All subjects were evaluated during the 1st semester of the year 2022, following the guidelines established by Genaro et al. in the MBGR orofacial myofunctional examination [25, 38, 39] (Fig. 3). Finally, the type of masticatory pattern was evaluated as bilateral alternating, bilateral simultaneous, unilateral preferential or chronic unilateral.
Fig. 3Evaluation of masticatory function (Genaro et al., 2009)
Video Recordings of three masticatory acts were made for each subject evaluated, using a cereal bar divided into 3 equal pieces of 2 cm2, a videorecording of the mandibular dynamics of the PMFA was also made for each subject, with 3 photographs of the occlusion in MIC (frontal, right lateral and left lateral) and upper arch plus lower arch in occlusal view. In addition, a postural photograph of the subject is taken for subsequent postural analysis.
2.2. Statistical analysis
After conducting the evaluations of the subjects, the functional and PMFA recordings, mouth and postural photographs, completing the bruxism self-report questionnaires of Fonseca's TMD, we proceeded to identify the masticatory pattern present in each subject of the sample. The data were tabulated in a specific Excel spreadsheet and processed through descriptive and inferential statistics using the Office 365-2022 Excel system.
3. Results
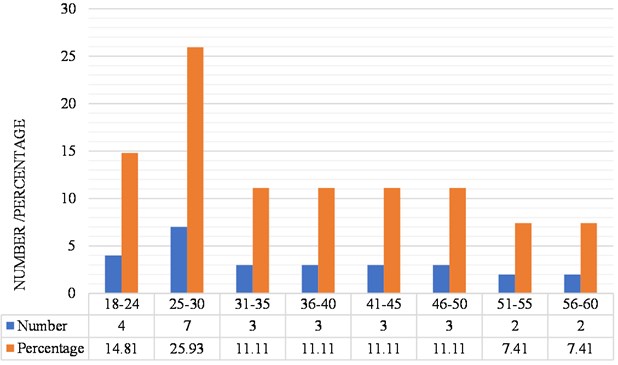
Regarding the age distribution of the study group of subjects with bruxism, the majority group is that of 25 to 30 years old with 7 subjects, representing 25.93 % of the total sample and the minority group is that of 51 to 54 and 56 to 60 years, both with 2 subjects representing 7.41 % of the sample (Fig. 4).
Fig. 4Distribution of subjects according to age of the subjects
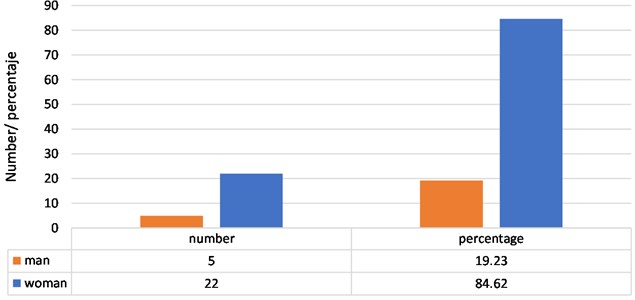
Regarding the distribution of subjects with bruxism of the sample, according to gender, it is have that 5 subjects are male, which represents 19.23 % of the sample and 22 are female, which represents 84.62 % of the sample (Fig. 5).
Fig. 5Distribution of subjects with bruxism, according to sex of the subjects
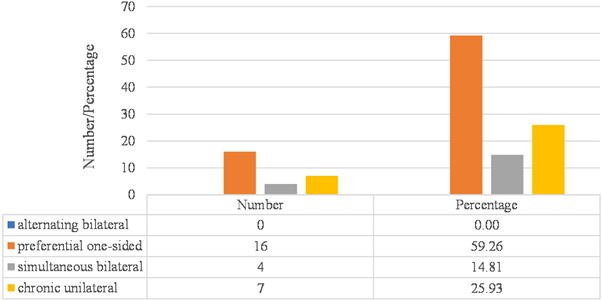
Regarding the distribution of subjects with bruxism in the sample, according to masticatory pattern, 100 % of the sample (27 cases) presented altered masticatory pattern, broken down as follows: 59.26 % corresponded to unilateral chewing (16 cases), 14.81 % to simultaneous bilateral chewing (4 cases), and 25.93 % to chronic unilateral chewing (7 cases) (Fig. 6).
Fig. 6Distribution of subjects with bruxism, according to their masticatory pattern
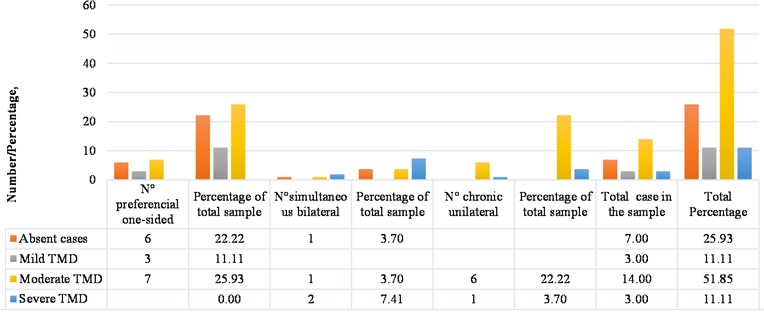
Regarding the distribution of the masticatory pattern of the subjects with bruxism in the present study, according to the degree of present TMD registered by application of the Fonseca questionnaire, 25.93 % of the total sample reported absent TMD (7 cases), and 74.07 % reported presenting TMD with some degree of compromise (20 cases). Of the group with absent TMD, 3.7 % of the total sample (1 case) presented a simultaneous bilateral masticatory pattern and 22.22 % of the total sample (6 cases) presented preferential unilateral chewing; of the 70.37 % of the total cases in the sample that reported TMD to some grade, 11.11 % of the total sample was observed with mild TMD (3 cases) that corresponded to a preferential unilateral chewing pattern; 51.85 % of the total sample reported a moderate TMD (14 cases) of which 25.93 % of the total sample (7 cases) presented a preferential unilateral pattern, 3.7 % of the total sample (1 case) presented a simultaneous bilateral pattern, and 22.22 % of the total sample (6 cases) had a chronic unilateral pattern; 7.41 % presented severe TMD (2 cases), of which 3.7 % of the total sample corresponded to a subject with a simultaneous bilateral chewing pattern (1 case), and the other 3.7 % of the total sample (1 case) of severe TMD corresponded to a subject with chronic unilateral chewing pattern (Fig. 7).
Fig. 7Distribution of cases with TMD, according to masticatory pattern of sample subjects
Regarding the degree of statistical correlation found in the sample between the presence of bruxism and the altered masticatory pattern, it is found that: 100 % of the cases in the sample, that is, all the individuals diagnosed with possible or probable bruxism present an altered masticatory pattern, 59.26 % preferential unilateral pattern, 14.61 % simultaneous bilateral pattern and 25.93 % chronic unilateral pattern. Therefore, the correlation value for this case is positive 1, which means that it is highly probable that a case of possible or probable bruxism presents an altered masticatory pattern.
Regarding the degree of statistical correlation found in the sample between the presence of TMD and altered masticatory pattern, 25.93 % of the cases in the sample did not present TMD according to Fonseca’s questionnaire, and 74.07 % of the cases in the sample presented some degree of TMD according to the Fonseca’s questionnaire. When applying the correlation test, a value of 0.93 was obtained, being a value close to 1, which means that it is very probable that a case of TMD presents an altered masticatory pattern
4. Discussion
The analysis of the masticatory pattern present in patients with possible and probable bruxism found that when chewing a standardized, hard, dry and fibrous food, 100 % of the cases in the sample presented a pattern different from that recognized as normal or physiological, that alternates bilaterally, quite the opposite to what was reported in a study of the masticatory pattern with healthy people or without complaints carried out by Moya et al. where 62 % of the participants [44], between 18 and 26 years old , presented a bilateral alternating pattern. The same occurs with the data obtained by Macedo et al. in a study with healthy people or without complaints, where 68 % of the participants (between 19 and 39 years old) presented a pattern of bilateral alternation [45].
The ideal physiological chewing should alternate bilaterally, as is the process of walking, with alternation of movements and joint displacements resulting from the muscular coordination between agonists and antagonists, considering for this all the information delivered by all the proprioceptive afferents from the receptors of the stomatognathic system and neighboring sense organs, including the postural tonic system. It is significant to see patients with alterations in the periodontal structure, dental problems, muscular alterations that present alterations in the masticatory pattern as pointed out by Inostroza-Allende et al., being one of the most treated functional difficulties by the professional speech therapist [46].
5. Conclusions
This pilot study confirms that chewing turns out to be a physiological, learned and peripheral mechanism that modulates the CNS, is permanently subject to peripheral stimuli and generates an adaptive response from its center that generates the chewing pattern. Therefore, an altered chewing pattern would generate adaptations at the central level that could not be physiological and therefore have a role in the generation of TMD and/or bruxism in those patients who have not correctly installed an alternative bilateral chewing pattern. Shifting the gaze towards the patient from the external to the internal would allow us to understand how their way of processing food could help find the solution in painful muscular conditions due to possible muscular overload or understand why dental wear has a different pattern than physiological wear that ends up compromising other neighboring structures such as the alveolar bone, or to understand why a patient with a diagnosis of TMD may end up with a lesion in their temporomandibular joint. The high statistical value found in the correlation between the present masticatory pattern and the condition of bruxism suggests that the patient's neuromuscular work during chewing should be evaluated in more detail and corrected if it presents any distortion. On the other hand, the high correlation found in the sample between the masticatory pattern and the presence of TMD was not total (0.934), which could be due to the different capacity of adaptation that individuals present to pain, muscle fatigue, or capacity of tissue regeneration.
It is necessary to carry out more studies with a larger sample to revalidate their results and start looking towards the installation of a more functional therapy for the management of bruxism and TMD, where teamwork with the speech therapist and other specialists in the area is a reality to obtain results that guarantee the improvement of patients and the stability of their oral health over time.
References
-
L. M. Ramírez-Aristeguieta, “Confusing dental paradigms: temporomandibular disorders, occlusion, and bruxism,” (in Spanish), Ustasalud, Vol. 7, pp. 132–143, 2008.
-
A. Manns et al., Stomatognathic System: Physiology and Its Clinical-Biological Correlations. (in Spanish), Madrid: Ripano, 2013.
-
Y. Yeerken, “Applicability of the CIELAB and CIEDE2000 formulae for detection of colour changes in colour-changeable chewing gum for evaluating masticatory function,” Journal of Clinical and Diagnostic Research, Vol. 11, No. 4, 2017, https://doi.org/10.7860/jcdr/2017/23950.9754
-
E. Aguirre-Siancas, “Neuroscientific bases of masticatory function and its effect on stress and cognitive functions,” (in Spanish), Revista Chilena de Neuropsiquiatría, Vol. 55, No. 1, pp. 9–17, 2017.
-
E. E. Aguirre-Siancas, “Bases neurocientíficas de la función masticatoria y su efecto sobre el estrés y las funciones cognitivas,” Revista chilena de Neuro-Psiquiatría, Vol. 55, No. 1, pp. 9–17, Apr. 2017, https://doi.org/10.4067/s0717-92272017000100002
-
Y. Ono, T. Yamamoto, K.-Y. Kubo, and M. Onozuka, “Occlusion and brain function: mastication as a prevention of cognitive dysfunction,” Journal of Oral Rehabilitation, pp. 624–40, Feb. 2010, https://doi.org/10.1111/j.1365-2842.2010.02079.x
-
I. Okayasu, O. Komiyama, N. Yoshida, K. Oi, and A. de Laat, “Effects of chewing efforts on the sensory and pain thresholds in human facial skin: A pilot study,” Archives of Oral Biology, Vol. 57, No. 9, pp. 1251–1255, Sep. 2012, https://doi.org/10.1016/j.archoralbio.2012.02.013
-
Y. Fukushima-Nakayama et al., “Reduced mastication impairs memory function,” Journal of Dental Research, Vol. 96, No. 9, pp. 1058–1066, Aug. 2017, https://doi.org/10.1177/0022034517708771
-
A. Marson, A. Tessitore, E. Sakano, and K. Nemr, “Efetividade da fonoterapia e proposta de intervenção breve em respiradores orais,” (in Portuguese), Revista CEFAC, Vol. 14, No. 6, pp. 1153–1166, Jun. 2012, https://doi.org/10.1590/s1516-18462012005000054
-
F. Susanibar, I. Marchesan, and D. D. A. Parra, Treatise on Orofacial Motor Evaluation. Madrid: EOS, 2014.
-
Elías Ernesto Aguirre-Siancas, “Memory and learning and its relation to chewing,” Revista Mexicana de Neurociencia, Vol. 4, pp. 351–354, 2014.
-
L. Malca, “Orofacial functional characteristics in young patients with inner open bite,” Master’s Thesis, Lima, Pontificia Universidad Católica del Perú, 2016.
-
W. A. Simões, “View of mandibular and maxillary growth,” (in Portuguese), Jornal Brasileiro de Ortodontia e Ortopedia Facial, Vol. 3, No. 15, pp. 9–18, 1998.
-
F. A. Torres Latorre and Y. M. Tenorio Cahuana, “Patrón de masticación según ángulo funcional de planas y prueba funcional de christensen y radue en niños,” (in Spanish), Revista Odontológica Basadrina, Vol. 6, No. 1, pp. 21–27, Jan. 2022, https://doi.org/10.33326/26644649.2022.6.1.1267
-
A. Zerón, “Bruxism and occlusal trauma. Multidisciplinary knowledge and interdisciplinary practice,” (in Spanish), Revista de la Asociacion Dental Mexicana, Vol. 75, No. 4, pp. 176–177, 2018.
-
Fernando Alberto Fuentes-Casanova, “Current knowledge for the understanding of bruxism. Literature review,” (in Spanish), Revista de la Asociacion Dental Mexicana, Vol. 75, No. 4, pp. 180–186, Sep. 2018.
-
Y. Hirano and M. Onozuka, “Chewing and attention: a positive effect on sustained attention,” BioMed Research International, Vol. 2015, pp. 1–6, 2015, https://doi.org/10.1155/2015/367026
-
J. Jimenez, “Importance of atypical swallowing in malocclusions,” (in Spanish), Odontología Sanmarquina, Vol. 19, No. 2, pp. 41–44, 2016.
-
Y. Morales, F. Neri, and J. Castellanos, “Physiopathology of nocturnal bruxism: Endogenous and exogenous factors,” (in Spanish), Revista de la Asociación Dental Mexicana, Vol. 72, No. 2, pp. 78–84, 2015.
-
C. Felício, M. Mazzetto, and C. Perri, “Masticatory behavior in individuals with temporomandibular disorders,” Minerva Stomatologica, Vol. 51, No. 4, pp. 111–120, 2002.
-
D. Garrigós, A. Garza, and J. Castellanos, “Bruxism: Beyond the teeth. An inter and multidisciplinary approach,” (in Spanish), Revista de la Asociación Dental Mexicana, Vol. 72, No. 2, pp. 70–77, 2015.
-
S. Schott Börger et al., “Métodos de evaluación del rendimiento masticatorio: Una revisión,” (in Spanish), Revista Clínica de Periodoncia, Implantología y Rehabilitación Oral, Vol. 3, No. 1, pp. 51–55, Apr. 2010, https://doi.org/10.4067/s0719-01072010000100009
-
J. Kim, J. Park, and J. Yim, “The effects of masticatory exercise using a gum on the cognitive function and stress,” Bioscience and Medical Research 2015, pp. 38–43, Aug. 2015, https://doi.org/10.14257/astl.2015.105.08
-
Y. Watanabe, H. Hirano, and K. Matsushita, “How masticatory function and periodontal disease relate to senile dementia,” Japanese Dental Science Review, Vol. 51, No. 1, pp. 34–40, Feb. 2015, https://doi.org/10.1016/j.jdsr.2014.09.002
-
K. F. Genaro, G. Berretin-Felix, M. I. B. C. Rehder, and I. Q. Marchesan, “Avaliação miofuncional orofacial: protocolo MBGR,” (in Portuguese), Revista CEFAC, Vol. 11, No. 2, pp. 237–255, Jun. 2009, https://doi.org/10.1590/s1516-18462009000200009
-
E. E. Castrillon, K.-L. Ou, K. Wang, J. Zhang, X. Zhou, and P. Svensson, “Sleep bruxism: an updated review of an old problem,” (in Spanish), Acta Odontologica Scandinavica, Vol. 74, No. 5, pp. 328–334, Jul. 2016, https://doi.org/10.3109/00016357.2015.1125943
-
R. Chávez, J. Castellanos, and A. Pacheco, “The dentoskeletal factor and nocturnal bruxism,” (in Spanish), Revista de la Asociación Dental Mexicana, Vol. 72, No. 2, pp. 85–91, 2015.
-
J. Lazaro, “Validation of the simplified anamnestic index of Fonseca for the diagnosis of temporomandibular disorders,” (in Spanish), Universidad Nacional Mayor De San Marcos, 2008.
-
S. J. Castellanos, “Bruxism. Notions and concepts,” (in Spanish), Revista de la Asociación Dental Mexicana, Vol. 72, No. 2, pp. 63–69, Mar. 2015.
-
C. C. Peck, “Biomechanics of occlusion – implications for oral rehabilitation,” Journal of Oral Rehabilitation, Vol. 43, No. 3, pp. 205–214, Mar. 2016, https://doi.org/10.1111/joor.12345
-
E. González, E. Midobuche, and J. Castellanos, “Bruxism, and tooth wear,” (in Spanish), Revista de la Asociación Dental Mexicana, Vol. 72, No. 2, pp. 92–98, 2015.
-
A. Díaz and K. Castañeda, “Evaluation of the effectiveness of diagnostic methods and treatment of bruxism: systematic review,” (in Spanish), Universidad Santo Tomás de Aquino, 2018.
-
C. Garcés, L. Godoy, A. Palacio, and M. Naranjo, “Action and influence of bruxism on the masticatory system; Literature review,” (in Spanish), Revista CES Odontología, Vol. 21, No. 1, 2008.
-
N. Cruz and M. González, “Self-reported bruxism questionnaire. Pilot study in northeastern Mexico,” (in Spanish), Interdisciplinaria, Vol. 36, pp. 217–232, 2019.
-
M. R.-A. Ramirez-Alvarez, “An analysis of axis II of the research diagnostic criteria (CDI/TTM) in an institutionalized elderly population in Mexico,” (in Spanish), Revista Tamé, Vol. 8, No. 23, pp. 908–912, 2019.
-
F. Lobbezoo et al., “International consensus on the assessment of bruxism: report of a work in progress,” Journal of Oral Rehabilitation, Vol. 45, No. 11, pp. 837–844, Nov. 2018, https://doi.org/10.1111/joor.12663
-
A. Nríquez, J. Balderas, D. García, and J. Castellanos, “Interdisciplinary assessment and management of bruxism,” (in Spanish), Revista de la Asociación Dental Mexicana, Vol. 72, No. 2, pp. 99–105, 2015.
-
S. Cortese and A. Biondi, “Relationship of parafunctional oral dysfunctions and habits with temporomandibular disorders in children and adolescents,” (in Spanish), Archivos Argentinos de Pediatría, Vol. 107, No. 2, pp. 134–138, 2009.
-
P. Planas, Neuro-Occlusal Rehabilitation. (in Spanish), Madrid: Amolca, 2008.
-
C. A. Rodrigues, M. O. Melchior, L. V. Magri, W. Mestriner Jr., and M. O. Mazzetto, “Is the masticatory function changed in patients with temporomandibular Disorder?,” Brazilian Dental Journal, Vol. 26, No. 2, pp. 181–185, Apr. 2015, https://doi.org/10.1590/0103-6440201300198
-
P. Bourdiol, M. Hennequin, M.-A. Peyron, and A. Woda, “Masticatory adaptation to occlusal changes,” Frontiers in Physiology, Vol. 11, Apr. 2020, https://doi.org/10.3389/fphys.2020.00263
-
E. Aguirre and S. Granados, “The deterioration of spatial memory and the role of the masticatory function during aging: a brief literature review,” British Journal of Medicine and Medical Research, Vol. 6, No. 12, pp. 1177–1185, Jan. 2015, https://doi.org/10.9734/bjmmr/2015/15779
-
S. Alvarado-Menacho, “Importance of simplified indices in the diagnosis of Temporomandibular Disorders,” (in Spanish), Revista Estomatologica Herediana, Vol. 28, No. 1, pp. 89–94, 2018.
-
M. P. Moya, K. Marquardt, and S. Olate, “Characterization of the masticatory function in university students,” (in Spanish), International Journal of Odontostomatology, Vol. 11, No. 4, pp. 495–499, Dec. 2017, https://doi.org/10.4067/s0718-381x2017000400495
-
P. F. A. Macedo and E. M. G. Bianchini, “Myofunctional orofacial examination: comparative analysis in young adults with and without complaints,” CoDAS, Vol. 26, No. 6, pp. 464–470, Dec. 2014, https://doi.org/10.1590/2317-1782/20142014015
-
F. Inostroza-Allende et al., “Masticatory function in Chilean young adults of both sexes,” (in Spanish), Revista Chilena Fonoaudiología, Vol. 19, 2020.
About this article
The authors have not disclosed any funding.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
The authors declare that they have no conflict of interest.
