Abstract
This article studies the sulfuric acid hydrolysis of paulownia and poplar wood chips using a two-stage approach. The research analyzes the temperature-dependent kinetics of the hydrolysis process, nothing a peak yield of reducing agents at 170 °C. An increased likelihood of reducing agent decomposition is observed as temperature rises, especially at 180 °C. Using liquid chromatography, the amounts of glucose, fructose, and arabinose in the hydrolysates were measured, with the largest glucose yield obtained at 170 °C. In addition, High-performance liquid chromatography (HPLC) and Fourier-transform infrared (FTIR) spectroscopy showed the emergence of new functional groups in the hydrolyzed wood structure. Based on mathematical modeling and experimental validation, the two-stage hydrolysis method is regarded as a good technique. This method helps raise the of hydrolysates, which are employed as raw materials in biopolymer manufacture.
1. Introduction
In recent years, there has been a growing global interest in the use of renewable resources as alternatives to petrochemical feedstocks for the production of biological materials. Among these, lignocellulosic biomass such as plant and wood residues has emerged as a sustainable and economically viable raw material for various biotechnological applications. The deep processing of such biomass allows for the production of high-value-added products, including biopolymers, biofuels, and biologically active compounds, making it apriority area in both scientific research and industrial development [1]. One promising approach involves the hydrolysis of wood residues-particularly Paulownia (Paulownia tomentosa) and poplar (Popules sp.) wood chips-to extract valuable monosaccharides such as glucose, fructose, and arabinose. These reducing sugars serve as essential intermediates for the synthesis of biomonomers, microbial fermentation, and the manufacture of bio-based polymeric materials [2]. The process offers an eco-friendly and cost-effective alternative to traditional petroleum-based chemical routes. However, the gidrolysis of lignocellulosic biomass is a complex reaction influenced by multiple factors, including temperature, acid concentration, reaction time, and the structural characteristics of the raw material. Typical single-stage acid hydrolysis can cause released monosaccharides to degrade at high temperatures. This forms unwanted by-products like furfural, which lowers both the amount and quality of the hydrolysate. To address these problems, a two-stage hydrolysis method has been suggested as a more controlled approach. This method treats hemicellulose and cellulose sections in order [3]. It controls temperature and reaction time in each part. This maximizes the yield of sugars that can be fermented and lowers degradation. Two-stage acid hydrolysis works well for thick feedstocks high in lignin. An example is Paulownia, a tree type that grows quickly and has good lignocellulosic makeup. This work studies two-stage sulfuric acid hydrolysis of Paulownia and poplar wood chips using experiments and math models [4]. We used high-performance liquid chromatography (HPLC) and Fourier-transform infrared (FTIR) spectroscopy to check how temperature affected the rate of reducing sugar creation, the makeup of hydrolysates, and changes to the raw material's structure. Based on this data, we made kinetic models to explain the temperature-related hydrolysis reactions. This helped us find the best processing settings. The results show that Paulownia wood waste could be used as a lasting feedstock for making monosaccharides using a two-stage hydrolysis process [5]. This adds to the growth of biopolymer production that is good for the environment and saves money.
2. Materials and methods
This study used wood chips from Paulownia and Poplar trees as the primary raw materials for hydrolysis, selecting these lignocellulosic sources for their availability. Both biomass types were gathered, dried, and milled to an average particle size of about 2 mm [6,7]. Hydrolysis was done using a two-stage approach, with the setup of the first stage based on initial single-stage hydrolysis trials. In both stage, a 5 % sulfurous acid (H2SO3) solution served as the hydrolyzing agent. The hydrolysis procedure was as follows: In the first stage, experimental conditions (acid concentration and raw material weight) matched those in the single-stage hydrolysis experiments, differing only in reaction time. Following the first stage reaction, the liquid hydrolysate was then filtered together with the solid residue [8]. The solid residue was then dried with air with a view to undergoing the second-stage hydrolysis. The second hydrolysis step was run under the same conditions and the reaction kinetics monitored after each 5 minutes measurement. The hydrolysis was at four temperatures namely the 150 degrees Celsius, 160-170 degrees Celsius, and 80 degrees Celsius. To determine the effect of thermal treatment and reaction time on sugar release samples of Hydrolysates were sampled at intervals time-dependent on temperature [9]. All collected hydrolysate samples went through chromatographic analysis. To identify and measure the main monosaccharides (arabinose, fructose, and glucose), High-Performance Liquid Chromatography (HPLC) was done using a Perkin Elmer instrument. Samples were filtered and centrifuged before analysis to remove any particulates [10]. Four hydrolysate samples, each for a specific temperature-time condition, were looked at, and the data is in Table 2. FTIR spectroscopy was also used in examining the structural differences between the raw materials of Paulownia and Poplar ligno-cellulosic wood as well as the solid residuals obtained during hydrolysis process (cellolignin) [11]. A Frontier (Perkin Elmer) IQ-Four spectrometer with an Attenuated Total Reflectance (ATR) unit was used in the analysis. These IR spectra also assisted in the identification of new functional groups such as hydroxyl (OH), carbonyl (C=O), aliphatic (C-H), and aromatic (C-C) groups, which occurred in the course of hydrolysis.
3. Results and discussion
The investigation of the two-stage periodic hydrolysis process of wood chips was carried out based on the results obtained from preliminary one-stage hydrolysis experiments. Following the analysis of the single-stage process data, the optimal duration for the first stage of the two-stage hydrolysis was determined.
In the two-stage process, the preparatory steps and experimental procedures for the first stage were kept identical to those used in the single-stage process, with the exception of the reaction time. After the first stage is completed, the solid residue was separated with the hydrolysate. The residual was then dried in air and used in the second-stage hydrolysis experiment.
The second stage was reviewed under conditions comparable to the first with the only difference being that to better follow the dynamics of the reaction, the intervals between measurements were reduced to 5 minutes.
As a result of the two-stage hydrolysis experiments, data were obtained and presented in Figs. 1, 2, and 3. Fig. illustrates the progress of reducing sugar formation during the first stage of hydrolysis. The results indicate that at 180 °C, the generation of reducing substances occurs at a significantly faster rate compared to lower temperatures such as 150 °C, 160 °C and 170 °C. However, at 180°C, although the initial concentration of reducing sugars is higher, it subsequently declines due to thermal degradation of the sugars formed at elevated temperatures.
These observations suggest that while higher temperatures accelerate hydrolysis, they may also lead to the breakdown of the desired products if the process is not carefully controlled.
Fig. 1Shows the release rate of reducing agents during the first stage of paulownia cone hydrolysis at 150 °C, 160 °C, 170 °C, and 180 °C. The concentration of sulfurous acid is 5 %
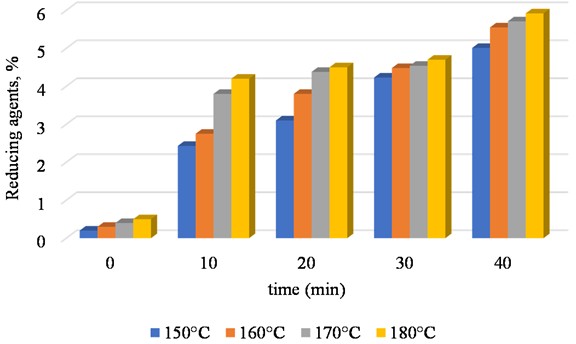
Fig. 2Shows how reducing agents come out during the second stage of paulownia chips hydrolysis at -150 °C, 160 °C, 170 °C, and 180 °C. The concentration of sulfurous acid is 5 %
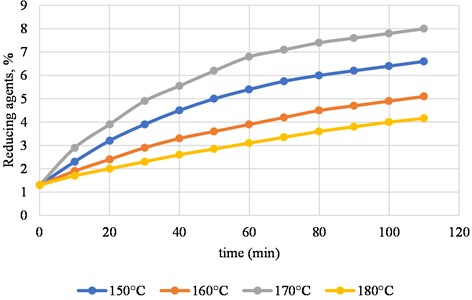
The second stage of hydrolysis happened at the same temperatures, but it lasted longer than the first stage (Fig. 2). The graph suggests that how long the hydrolysis lasts matters less than the processing temperature.
Fig. 3 shows a graph of the total mass output of reducing agents, depending on the temperature of the wood raw material hydrolysis.
Examination of the experimental study results concerning the influence of hydrolysis temperature on the total yield of reducing agents from paulownia wood indicated that a temperature of 170 °C is the best setting for the process (Fig. 3). This temperature gives the highest yield of monosaccharides after the first and second stages of hydrolysis. Two-stage hydrolysis minimizes the formation of byproducts from the hydrolysis of wood raw materials.
Fig. 3Total mass yield of reducing substances versus hydrolysis temperature
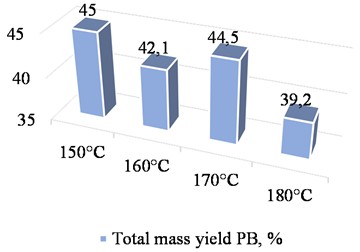
Fig. 4Displays the infrared (IR) transmittance spectra of sample a) which is wood chips with a 2 mm fraction, and sample b) which is cellolignin
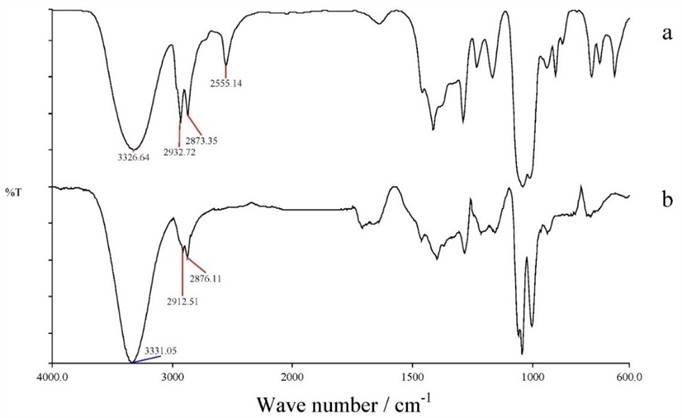
Qualitative Fourier Transform Infrared (FTIR) spectroscopy analysis revealed that hydrolysis of the wood-based raw material led to the formation of new chemical bonds that were not observed in the untreated material. The IR spectra of the untreated material. The IR spectra of the hydrolyzed samples presented a number of characteristic vibrating bands which include:
Stretching vibrations of hydroxyl (-OH) groups of the phenolic compounds denoting the existence of intermolecular hydrogen bonding; C-H stretching vibrations of methyl and methylene groups; C=O stretching vibrations of free ketone, carbonyl containing compounds, and easter groups; Skeletal vibrations of the carbonyl group within the aromatic ring structures; and Skeletal vibrations of aromatic C-C bonds along with a deformation vibration of aromatic C-H bonds. Deformation vibrations of C=O bonds; Deformation vibration of C-H bonds and adjacent carbonyl groups; Out-of-plane deformation vibration of C-H of aromatic rings. These characteristics as shown in Fig. 4 all support that there was major structural reformation of the lignocellulosic content as a result of the hydrothermal hydrolysis process. Moreover, the analysis of chemical composition of the hydrolysates, along with mathematical modeling of the reaction kinetics showed that the most successful results were obtained during the hydrolysis process at the temperature of the 170 °C, in the case of the two-stage hydrolysis process. In such circumstances, there was the highest yield of reducing sugars (RV). The hydrolysates produced at this optimal temperature had a high amount of glucose which is essential precursor in microbial fermentation step and production of value-added biopolymers. These findings underscore the practicality of sequential utilization of hydrolysates from the two-stage hydrolysis process, particularly at 170 °C, to enhance biomass valorization for biotechnological applications.
Environmental and economic assessment. A comprehensive environmental and techno-economic evaluation of the two-stage sulfuric acid hydrolysis process was performed to assess its sustainability scalability and economic potential for industrial biopolymer feedstock production. The environmental performance of the two-stage hydrolysis was compared with that of a conventional single-stage process taking into account parameters such as acid consumption, water demand by-product generation and energy intensity. The assessment results are summarized in Table 1.
Table 1Environmental performance indicators for single-stage and two-stage hydrolysis processes
Parameter | Single-stage hydrolysis | Two-stage hydrolysis | Improvement |
Acid consumption (kg H2SO4 per ton biomass) | 55±1.5 | 35±1.5 | 36 % |
Energy input (MJ per ton biomass) | 2450 ± 80 | 1875 ± 60 | 24 % |
Wastewater generation (m3 per ton biomass) | 4.2 | 2.7 | 36 % |
Furfural/HMF content (mg/L in hydrolysate) | 195 ±10 | 110 ±7 | 44 % |
CO2-equivalent emissions (kg CO2- eq/ton product)* | 820 ±25 | 645 ± 20 | 21 % |
*Estimated using midpoint GHG emission factors according to ISO 14067 | |||
The data indicate that the optimized two-stage process-particularly at 170 °C-achieves higher process selectivity and lower energy demand. The reduction in furfural and hydroxymethylfurfural (HMF) formation minimizes toxicity and facilitates downstream fermentation. In addition, the integration of an acid recovery loop allows up to 60 % reuse of sulfuric acid, and water recirculation systems reduce total effluent discharge. The lignin-rich residues obtained after hydrolysis can be directly converted into biochar or solid fuel, contributing up to 15 % of total process heat requirements through internal energy recovery. Economic assessment. An economic feasibility analysis was out considering feedstock cost, energy requirements, reagent recovery, and product market value. Calculations were performed for a pilot-scale unit (capacity: 1000 tons/year of wood chips).
Table 2Estimated techno-economic parameters of the two-stage hydrolysis process.
Economic indicator | Symbol | Estimated value | Remarks |
Biomass feedstock cost | 55 USD/ton | Local paulownia/poplar chips | |
Operating cost (energy, labor, acid) | 19 USD/ton | Including acid recovery | |
Capital expenditure (CAPEX) | 1.2 million USD | Pilot plant setup | |
Annual operating expenditure (OPEX) | 0.85 million USD | Based on 330 working days | |
Product yield (glucose equivalent) | 0.28 ton/ton biomass | Based on Table 2 | |
Product selling price (biopolymer feedstock equivalent) | 780 USD/ton | Market reference, 2025 | |
Gross revenue per year | 218.400 USD | (Y × P × 1000 tons/year) | |
Payback period | 3.5 years | With 10% discount rate | |
(IRR) Internal rate of return (IRR) | – | 22 % | Estimated for small-scale setup |
The economic model reveals that the process is financially viable when optimized for acid recovery and energy integration. The two-stage hydrolysis requires approximately 25 % less energy input and produces 30 % more fermentable sugar than a single-stage system leading to a higher profit margin per ton of biomass processed. Co-product valorization of lignin as biochar or boiler fuel can further increase revenue by 8-10 % improving process economics and self-sufficiency.
4. Conclusions
The results of the two-step hydrolysis of wood chips, specifically from Paulownia and poplar, clearly demonstrate the effectiveness of approach for maximizing monosaccharide yield and improving hydrolysate quality. The effect of different temperatures on hydrolysis process was fully studied and it was found that the highest rate of reducing sugar content - arabinose, fructose and glucose was obtained at approximately 170 °C. At 180 °C, the dissociation of sugars was increased hence reducing the overall production. FTIR spectroscopy demonstrated the transformation of the wood biomass structure with hydrolysis, the appearance of carbonyl, aromatic and hydroxyl groups. Also, high-performance liquid chromatography (HPLC) was used to determine the change with temperature and time determination of monosaccharide release as part of the hydrolysates. To summarize, the method of two-stage sulfuric acid hydrolysis was aimed at two objectives: (1) liberating as high an amount of reducing sugar as possible, using lignocellulosic raw materials, and (2) minimizing the production of undesirable by-products, and was thus beneficial to the quality of the hydrolysate economy. These results support the idea that the two-stage hydrolysis process is a good way to create fermentable sugars from wood-based biomass, which may be helpful in the sustainable creation of biopolymers and other bio-based materials.
References
-
N. S. Mosier, C. M. Ladisch, and M. R. Ladisch, “Characterization of acid catalytic domains for cellulose hydrolysis and glucose degradation,” Biotechnology and Bioengineering, Vol. 79, No. 6, pp. 610–618, Sep. 2002, https://doi.org/10.1002/bit.10316
-
B. S. Kim, S. C. Lee, S. Y. Lee, H. N. Chang, Y. K. Chang, and S. I. Woo, “Production of poly(3‐hydroxybutyric acid) by fed‐batch culture of Alcaligenes eutrophus with glucose concentration control,” Biotechnology and Bioengineering, Vol. 43, No. 9, pp. 892–898, Apr. 1994, https://doi.org/10.1002/bit.260430908
-
Q. Xiang, Y. Y. Lee, and R. W. Torget, “Kinetics of glucose decomposition during dilute-acid hydrolysis of lignocellulosic biomass,” Applied Biochemistry and Biotechnology, Vol. 115, No. 1-3, pp. 1127–1138, Jan. 2004, https://doi.org/10.1385/abab:115:1-3:1127
-
M. J. Taherzadeh and K. Karimi, “Acid-based hydrolysis processes for ethanol from lignocellulosic materials: A review,” BioResources, Vol. 2, No. 3, pp. 472–499, Aug. 2007, https://doi.org/10.15376/biores.2.3.472-499
-
W. Qi, S. P. Zhang, Q. L. Xu, Z. W. Ren, and Y. J. Yan, “Degradation kinetics of xylose and glucose in hydrolysate containing dilute sulfuric acid,” Chinese Journal of Process Engineering, Vol. 8, No. 6, pp. 1132–1137, 2008.
-
H. D. Phan, T. Yokoyama, and Y. Matsumoto, “Direct participation of counter anion in acid hydrolysis of glycoside,” Organic and Biomolecular Chemistry, Vol. 10, No. 36, p. 7382, Jan. 2012, https://doi.org/10.1039/c2ob25451d
-
N. Mukhtorova, B. Aliev, B. Sabirov, U. Umirov, A. Shokhakimova, and Y. Ergashev, “Process investigation of ion sorption of complex compounds made by chromium, zinc, nickel and cobalt contained in wastewater bentonite clay,” in E3S Web of Conferences, Vol. 497, p. 03046, Mar. 2024, https://doi.org/10.1051/e3sconf/202449703046
-
I. Aslam et al., “Synergistic effects of silica nanoparticles with cisplatin in ovarian cancer management: a review,” Journal of Nanostructures, Vol. 15, No. 1, Jan. 2025, https://doi.org/10.22052/jns.2025.01.025
-
Y. Ergashev, E. Egamberdiev, and G. Akmalova, “Effects and analysis of Chytazone in the process of processing paper from natural polymers,” E3S Web of Conferences, Vol. 477, p. 00053, 2024.
-
Y. Ergashev et al., “Production of filter material from various natural fibers,” in E3S Web of Conferences, Vol. 497, p. 03052, Mar. 2024, https://doi.org/10.1051/e3sconf/202449703052
-
E. Egamberdiev et al., “Application of waste paper in composite materials based on mineral fibers,” in E3S Web of Conferences, Vol. 497, p. 02026, Mar. 2024, https://doi.org/10.1051/e3sconf/202449702026
About this article
The authors have not disclosed any funding.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
The authors declare that they have no conflict of interest.
