Abstract
This work presents the development of an innovative laboratory installation for the regeneration of spent zeolites used in the purification of natural and wastewater from heavy metal ions and other pollutants. The technological scheme of the installation is described, enabling efficient restoration of the dynamic activity of zeolite sorbents, which ensures their multiple reuse and reduces dependence on imported synthetic sorbents. The possibility of reusing eluates obtained during regeneration as components of electrolytes for electroplating processes is considered. The technical characteristics of the installation are provided, and the application areas of regenerated zeolites are described, including gas stream purification, fuel drying, and transformer oil dehydration. The expected effect of implementation is the optimization of regeneration modes, reduction of waste, and an increase in the environmental safety of production processes.

Highlights
- An innovative pilot laboratory setup was developed for efficient oxidative–reductive regeneration of spent zeolites.
- The installation restores the dynamic activity of zeolite sorbents, enabling their multiple reuse and reducing waste generation.
- The regenerated zeolites can be used in gas purification, fuel drying, and transformer oil dehydration, improving environmental sustainability.
1. Introduction
In the modern world, environmental issues have become an integral part of almost every aspect of human activity. All areas – industry, everyday life, and even leisure – are now closely linked to ecological problems and the need to protect the environment [1-3]. More and more people recognize that the issues of rational use of natural resources, minimizing emissions of harmful substances, and ensuring environmental safety are directly connected to the quality of life for present and future generations [4].
The existence of living organisms is possible only if the parameters of their environment remain within ranges that are favorable for each particular species. The ecological characteristics of a habitat must correspond to the physiological and biochemical needs of the organism, without provoking stress or pathological reactions. While the optimal environmental parameters vary for different species, a fundamental requirement is that concentrations of harmful and hazardous factors should not exceed permissible threshold values. Exceeding these values often results in deteriorating health, decreased biological productivity, and even the extinction of certain populations [5].
The quality of inhaled air is of particular importance for the survival of all living beings. It is crucial not only to maintain the optimal ratio of basic atmospheric components – nitrogen, oxygen, and carbon dioxide – but also to monitor the content of impurities such as dust, soot, sulfur and nitrogen oxides, chlorine compounds, dioxins, volatile organic compounds, and other pollutants [6]. This problem is especially acute in large cities and industrial centers, where anthropogenic pressure leads to an unfavorable ecological situation, and air quality often fails to meet the established sanitary standards necessary for comfortable and safe living [7, 8].
With the growth of urbanization and industrial production, the need for regular air purification and disinfection in public and industrial buildings has become increasingly relevant. Various types of filters are used today: from absolute filters for fine purification to carbon and bactericidal filtration systems. However, despite their widespread use, each technology has certain technological and operational limitations, such as the need for frequent replacement of filter materials, a limited range of captured pollutants, and high maintenance costs [9].
Modern requirements for environmental protection and the growing volume of waste dictate the need for new technological solutions that provide not only effective treatment of emissions and wastewater but also the utilization of various types of waste. The most promising approaches include the transition to low-waste or zero-waste technologies, optimization of resource-saving processes, and improving the efficiency of treatment facilities. The implementation of such technologies significantly reduces anthropogenic impact on the biosphere by lowering raw material consumption, decreasing the volume of pollutants released into the environment, and enhancing the environmental safety of production processes [10].
A review of current scientific literature demonstrates the high demand for research aimed at improving methods for the regeneration of molecular sieve adsorbents, especially natural zeolites. Particular attention is paid to their use in purifying natural gas from sulfur compounds, as well as finding new areas for the application of regenerated zeolites, which is especially relevant in the context of increasing the efficiency of purification processes and promoting resource reuse [11-14]. Addressing these issues is of both scientific and practical importance for modern industries focused on the implementation of innovative and environmentally safe technologies.
2. Results and discussion
Based on the results of our previous research and literature analysis, it has been determined that the decrease in the adsorption capacity of zeolites during their use in adsorption units for natural gas purification is caused by the accumulation of high-molecular-weight products and carbonaceous deposits on the surface and within the pores of the sorbents. These deposits are formed as a result of cracking and pyrolysis of components present in raw gas on the acidic sites of the sorbents [15, 16].
At gas processing plants in Uzbekistan, zeolites are widely used for hydrogen sulfide removal and natural gas dehydration. Once their adsorption capacity declines, the zeolites are discharged and disposed of as waste. The lack of domestic production of synthetic zeolites in the Republic, along with the limited service life of these materials, significantly increases the cost of the process due to the need for annual purchases of imported sorbents.
Therefore, the development of technology for producing new, efficient sorbents based on zeolitic waste from the petroleum refining industry of the Republic is a relevant issue for the dehydration and purification of natural gas.
In line with the above considerations, a technological scheme of a new innovative pilot laboratory installation for the regeneration of zeolitic waste has been developed, which is shown in Fig. 1.
Based on the developed technological scheme, a technical assignment was issued for the fabrication of a pilot laboratory installation to conduct experiments aimed at determining the optimal zeolite regeneration mode.
Fig. 1Technological scheme of the pilot laboratory installation: 1) reactor; 2) steam generator; 3) water tank; 4) compressor; 5) control and temperature monitoring panel; 6) air receiver; 7) gas meter; 8) thermocouple pocket; 9) manometer; 10) flask for neutralizing exhaust gases; 11) condenser
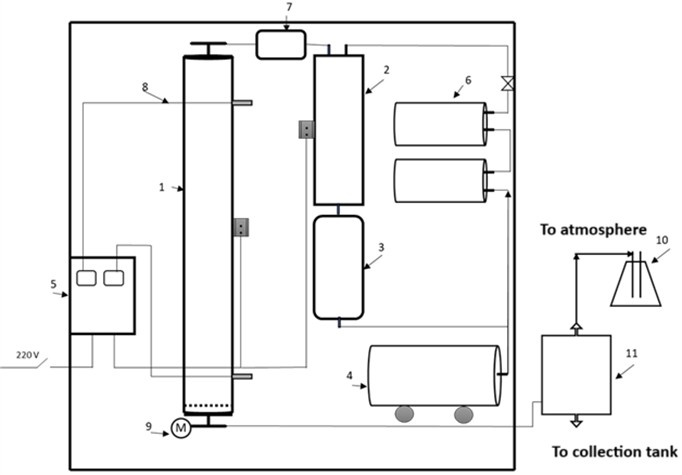
Fig. 2General view of the laboratory unit for oxidative-reductive regeneration of spent zeolites. The photograph was taken in March-April 2024 at “Neftgazinnovatsiya” LLC, Tashkent, Uzbekistan, where the prototype of the industrial-scale installation was developed

2.1. Chemical processes during zeolite regeneration
The primary chemical process occurring during operation of the laboratory unit is the oxidative–reductive regeneration of spent zeolites. Contaminant residues, mainly carbon (C), hydrogen (H), and sulfur (S) in a coke-like form, react with oxygen from the air to form volatile species (CO2, SO2, H2O, CO) that are removed from the transport pores of the zeolite: CnHmSz+O2 = nCO+mCO2+kSO2+8H2.
Analysis of the exhaust gas composition shows that these components are typically present in the following proportions: C – 82-86 %, S –8-12 %, H – 3-4 %. Based on these results, the mathematical framework of mass-transfer theory can be used to quantify the number and structure of the transport pores of the zeolite and their role in the adsorption process.
In the course of the research, a technological scheme was developed and a pilot laboratory installation was fabricated for modeling and thoroughly studying various modes of regeneration of spent zeolites used in adsorption processes for natural gas purification. The installation makes it possible to simulate real operating conditions of the sorbents, and its design is intended for restoring the dynamic activity of zeolitic materials when processing batches of 4-5 kg per cycle. This solution provides the flexibility to vary process parameters and enables prompt analysis of the efficiency of selected regeneration modes. The technical specifications and main parameters of the unit are presented in Table 1, demonstrating that the laboratory model meets the requirements necessary for practical application of this technology in industry.
Table 1Technical specifications of the pilot laboratory installation
Technical parameter | Value |
Regeneration furnace (activation reactor) | |
Working pressure range, MPa | 0-0.06 |
Reactor operating temperature range, °C | 50-850 |
Temperature setting increment, °C | 1 |
Temperature control relative error, °C | ±5 |
Reactor material | Stainless steel |
Wall thickness, mm | 3-4 |
Power consumption, kW | 4 |
Dimensions (H×D), cm | 100×7 |
Weight, kg | 20 |
Operating mode | Cyclic |
Water tank for steam generation: | |
Material | Stainless steel |
Volume, cm3 | 1500 |
Steam generator: | |
Material | Stainless steel |
Volume, cm3 | 1500 |
Outlet steam temperature, °C | 130-140 |
Steam pressure, MPa | 0.02 |
Capacity, cm3/h | 1500 |
Automatic temperature control device | 220 V, error ±1 °C |
Thermocouple | Chromel-Alumel |
Manometers | Reference (0-0.5 MPa) or digital type |
Technical Parameter | Value |
2.2. Description of installation components
1) Activation reactor (specifications provided in the table).
2) Steam generator made of stainless steel with a volume of 1500 cm3 and an outlet steam temperature of 130-140 °C.
3) Water tank made of stainless steel with a volume of 1500 cm3; A reference manometer for measuring pressure in the tank and a capillary copper tube for maintaining a precise water feed rate to the steam generator; steam pressure is maintained at 0.02 MPa.
4) Compressor for supplying air.
5) Control and temperature monitoring panel.
6) Air receiver.
7) Gas meter for measuring air flow.
8) Thermocouple pocket for temperature measurement.
9) Manometer for pressure measurement.
10) Flask with alkali for neutralizing exhaust gases.
11) Condenser.
Air from the compressor is supplied to the steam generator (2), where it is heated to 280-300 °C. From the steam generator, the heated air flows into the activation reactor (1), which is filled with the sorbent. The regenerated steam-gas mixture from the reactor passes into the condenser (11), where the water component is cooled, condensed, and collected in a reservoir, while the regeneration gases are passed through neutralization flasks (10) and discharged into the atmosphere.
The installation also provides for regeneration using water steam, which is generated by feeding water under pressure from the tank (3) through a capillary into the steam generator (2).
The installation, designed according to the developed technological scheme, enables the acquisition of experimental data and the selection and optimization of process parameters for the optimal regeneration mode of zeolite. The expected effect of the installation will be determined after studying the restoration of the dynamic activity of zeolite and after testing various operating modes, which will help reduce the Republic’s dependence on imported synthetic zeolites.
Areas of application for regenerated zeolite include use as filter-driers, in transformer oil regeneration, purification of gas streams such as natural and associated petroleum gas from sulfur compounds, mercaptans, and carbon dioxide, as well as air purification from carbon dioxide before air separation into nitrogen and oxygen, and drying of jet and diesel fuels.
3. Additional requirements
The installation must be vibration-resistant. The finished product, its components, and both raw and operational materials must comply with quality and completeness requirements established by relevant standards or technical specifications.
4. Conclusions
As a result of the research and the development of the innovative laboratory installation, the possibility of effective regeneration of spent zeolite sorbents with restoration of their dynamic activity was demonstrated. The proposed technological scheme ensures optimal conditions for zeolite regeneration, allowing their multiple reuse in various technological processes for water and gas purification. This not only reduces costs associated with purchasing imported sorbents but also addresses key environmental challenges related to waste disposal and minimization of negative environmental impact. The experimental data obtained confirm the prospects and practical significance of the developed installation for the oil and gas, energy, and other industrial sectors. The implementation of this technology may become one of the effective solutions for enhancing the ecological and resource sustainability of industrial enterprises in the Republic of Uzbekistan.
References
-
E. V. Kondratyuk, S. M. Maltseva, D. A. Stroganov, E. A. Ryabkova, and I. S. Trubina, “The use of natural zeolites as stationary filters for disinfection and purification of indoor air,” Siberian Journal of Life Sciences and Agriculture, Vol. 14, No. 3, pp. 172–191, Jun. 2022, https://doi.org/10.12731/2658-6649-2022-14-3-172-191
-
Y. Shadimetov and D. Ayrapetov, “Impact of transportation pollutants on the environment and human health,” in E3S Web of Conferences, Vol. 563, p. 03096, Aug. 2024, https://doi.org/10.1051/e3sconf/202456303096
-
E. N. Boyarov, S. V. Abramova, and D. A. Gershinkova, “Human security in the light of the current climate agenda,” Siberian Journal of Life Sciences and Agriculture, Vol. 13, No. 1, pp. 111–133, Feb. 2021, https://doi.org/10.12731/2658-6649-2021-13-1-111-133
-
E. W. Chu and J. R. Karr, “Environmental impact: concept, consequences, measurement ☆,” in Reference Module in Life Sciences, Elsevier, 2017, https://doi.org/10.1016/b978-0-12-809633-8.02380-3
-
J. D. Berman, “Air pollution and health-new advances for an old public health problem,” JAMA Network Open, Vol. 7, No. 3, p. e2354551, Mar. 2024, https://doi.org/10.1001/jamanetworkopen.2023.54551
-
Y. A. Izrael, Ecology and Control of the State of the Natural Environment. Moscow: Gidrometeoizdat, 1984.
-
O. V. Kalugina, T. A. Mikhailova, L. V. Afanasyeva, and O. V. Shergina, “Activity and isozyme composition of peroxidase in Scots pine (pinus sylvestris L.) needles effected by technogenic emissions from various enterprises and vehicles,” Siberian Journal of Life Sciences and Agriculture, Vol. 13, No. 1, pp. 11–34, Feb. 2021, https://doi.org/10.12731/2658-6649-2021-13-1-11-34
-
Y. Shadimetov and D. Ayrapetov, “Current issues in waste management in hot climate conditions,” Iop Conference Series Earth and Environmental Science, Vol. 1420, No. 1, p. 012005, 2024.
-
A. Yang, “The cost of clean air: a price analysis of air filtration technology,” arXiv:2208.06041, Jan. 2022, https://doi.org/10.48550/arxiv.2208.06041
-
C. S. Ferreira Fernandes, F. Alves, and J. Loureiro, “Sustainable futures: from causes of environmental degradation to solutions,” Discover Sustainability, Vol. 5, No. 1, Apr. 2024, https://doi.org/10.1007/s43621-024-00242-1
-
S. A. Mutalov, M. M. Niyazova, and D. B. Niyazov, “Regeneration of spent zeolites in the gas processing industry,” Universum: Chemistry and Biology, Vol. 11, No. 2, pp. 32–34, 2019.
-
C. Kassargy, S. Awad, G. Burnens, G. Upreti, K. Kahine, and M. Tazerout, “Study of the effects of regeneration of USY zeolite on the catalytic cracking of polyethylene,” Applied Catalysis B: Environmental, Vol. 244, pp. 704–708, May 2019, https://doi.org/10.1016/j.apcatb.2018.11.093
-
W. C. Kwak, Y. J. Oh, S. K. Kang, A. H. Lee, S. M. Jung, and P. S. Lee, “Regeneration of zeolite membranes deactivated by condensable molecules,” International Journal of Greenhouse Gas Control, Vol. 119, p. 103731, Sep. 2022, https://doi.org/10.1016/j.ijggc.2022.103731
-
E. Katsou, S. Malamis, M. Tzanoudaki, K. J. Haralambous, and M. Loizidou, “Regeneration of natural zeolite polluted by lead and zinc in wastewater treatment systems,” Journal of Hazardous Materials, Vol. 189, No. 3, pp. 773–786, May 2011, https://doi.org/10.1016/j.jhazmat.2010.12.061
-
M. I. A. Makhamadjanov and Z. Alimova, “Studying the dynamic characteristics of zeolites used in adsorption cycles for natural gas desulfuration,” in Fifteen International Conference on Thermal Engineering: Theory and Applications, May 2024.
-
M.-I. A. Makhamadjanov, Z. K. Alimova, R. N. Akhmatjanov, K. I. Magdiev, and T. Samataev, “Recovery of zeolite waste for reuse during cleaning natural gases,” in Problems in the Textile and Light Industry in the Context of Integration of Science and Industry and Ways to Solve Them: PTLICISIWS-2, Vol. 3045, p. 060032, Jan. 2024, https://doi.org/10.1063/5.0197342
About this article
The authors have not disclosed any funding.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
The authors declare that they have no conflict of interest.
